BIOMEX.
Empowering scientists.
Watch the video now and understand
how and why we are empowering scientists.

Biospecimens & Services.
For Life Sciences & Diagnostics.
Unlock the potential of your research with our high-quality biospecimens and expert services
Life Sciences Diagnostics


Your full-service provider for Life Sciences.
Achieve maximum results in your research endeavors with our all-inclusive solutions and expert guidance
Biospecimens Services ApplicationsBIMEX – Empowering Scientists
Diagnostic: Sample Sourcing & Full Service – Discover Europe’s largest inventory for human raw materials with more than 600,000 human biospecimens and 6,000 units of disease state plasma in stock. With our strategically located donation centers in Germany and Cameroon, extensive network of trusted partners, and state-of-the-art laboratories, we are well positioned to source and process even uncommon samples.
In our donation centers we produce 15,000L of negative plasma per year and have a reliable pool of donors.
For over 30 years, we’ve committed to delivering top-notch human raw material and reliable services to our valued customers.
Contract Research Organisation IVD (CRO) – As an IVD only CRO, we provide in the area of clinical performance all studies testing Annex I parameters, such as diagnostic sensitivity, diagnostic specificity, positive and negative predictive value, likelihood ratio, and expected values in normal and affected populations. We guarantee the highest quality along the entire process – and the best chances for an approval of the assay under EU IVDR, but also TGA and WHO. Our offer includes a study plan, ethics approvals, all required fresh and frozen samples, lab work, data analysis and a final report.
Life Sciences: Biospecimens, Biomarker Services & Applications – BIOMEX is on track to provide human biosamples and premium services to the entire life sciences industry! Put us to the test with your request – you will gain speed and competitive advantage through our high availability and flexibility. Plan your processes reliably through our high quality standards and focus on your core business with us as your experienced and agile full-service provider!
We’re discovering new opportunities and solving challenges for a better future.
Human biosamples that make the difference: We offer you the best conditions to meet your diagnostic challenges.
BINEWS
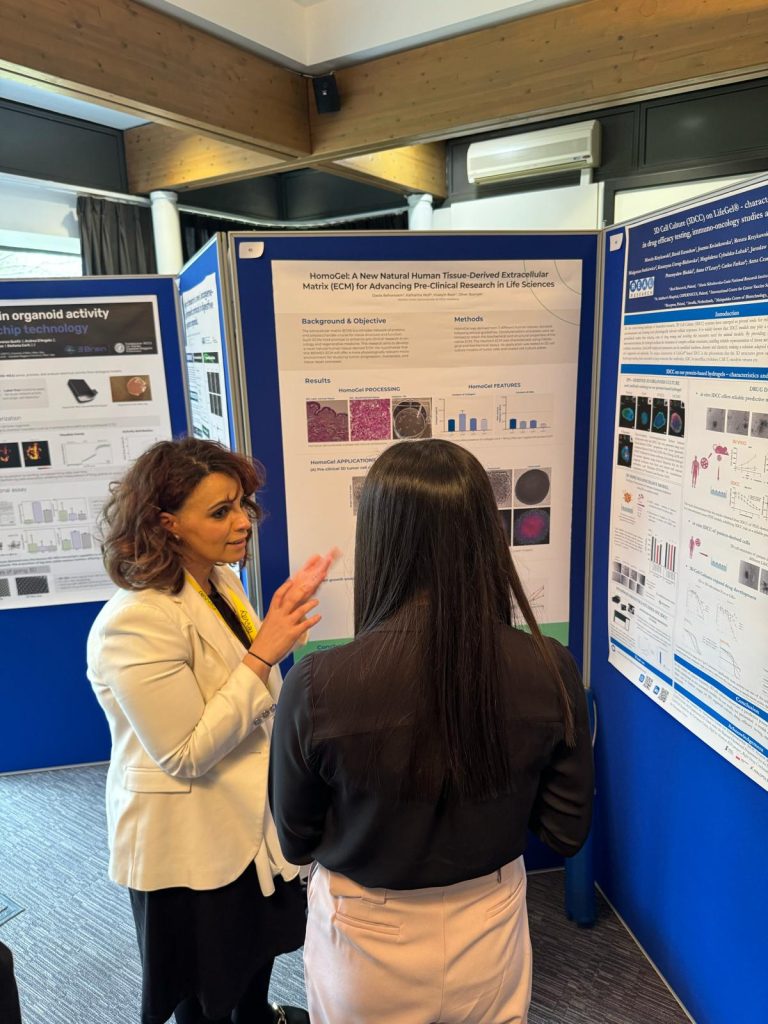
BIOMEX presents: Human ECM for cancer and stem cell research!
BIOMEX presents its innovative project: Human ECM for cancer and stem cell research at the World Organoid Research Day conference WORD 2024 at the Hinxton Hall Conference Centre in Hinxton,
BIOMEX at CACLP 2024 in China
From March 16 to 18, BIOMEX attended CACLP 2024 with its own booth, N3-0828. The
BIOMEX provides Matched Sets from healthy donors
Discover the impact of anticoagulants on test results with our matched sets sourced from healthy
BIOMEX presents: Human ECM for cancer and stem cell research!
BIOMEX presents its innovative project: Human ECM for cancer and stem cell research at the
Sampleshop
BIOMEX provides samples from existing donor collection centers and collaborates with many well respected laboratory facilities under strict ethical standards.
We routinely receive 5,000 remnant lab samples each month and have access to more than 100,000 samples per month. Our inventory contains more than 600,000 patient samples. Please take a moment to access our website:
Considering the chance that our inventory does not fulfill your needs, we encourage you to contact us to discuss acquiring these prospectively.

„Every brilliant experiment, like every great work of art, starts with an act of imagination.“

We want you!
Would you like to work in an environment in which your ideas are valued? Do you want to be part of a team that gives you the opportunity to contribute and grow?
Then come to BIOMEX! Our day-to-day work is characterized by respectful cooperation – because it is our employees who are our strength and key to success. We have a dynamic structure with short decision-making paths and are looking for people who act responsibly.
Diverse, exciting, and challenging tasks all await you at BIOMEX!