Performance Evaluation for Diagnostics
Performance Evaluation for In Vitro Diagnostics
Do you want to develop an in vitro diagnostic and bring it to market? To do so, your product must meet certain performance requirements. In Europe, for example, performance is evaluated in accordance with the European In Vitro Diagnostics Regulation 2017/746 (IVDR), which covers three areas: Scientific validity, analytical performance, and clinical performance. In addition, according to the IVDR, you must evaluate performance both before and after market launch:
Scientific validity means that an analyte is associated with a specific clinical condition or physiological state (IVDR, Article 2 (38)).
Analytical performance refers to the ability that a particular analyte can be correctly detected or measured (IVDR, Article 2 (40)).
Clinical performance describes the ability to provide results that correlate with a specific clinical condition or physiological or pathological process or state (IVDR, Article 2 (41)).
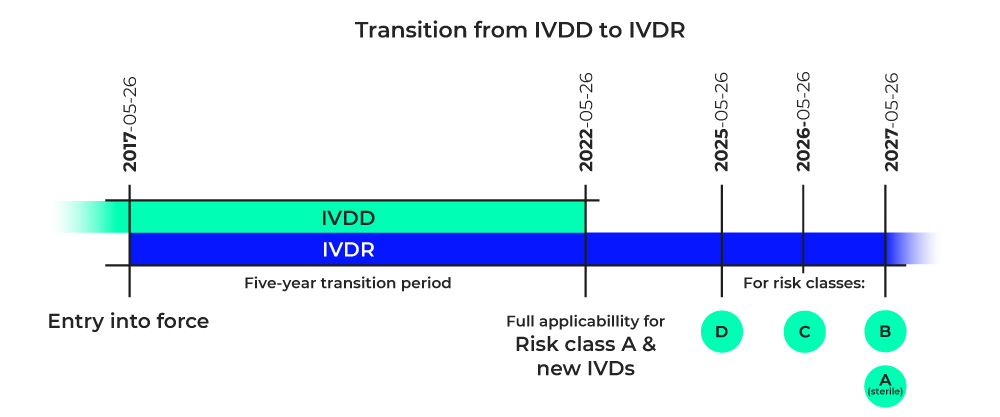
Before market introduction, manufacturers must identify all available data on the product, its intended use and safety, and fill data gaps in order to assess performance. Outstanding issues require the conduct of appropriate studies (IVDR, Annex XIII, Part A, 1.2).
After a product has been launched, manufacturers should proactively collect and evaluate performance and scientific data resulting from the product’s use to ensure safety, performance, and scientific validity throughout the product’s lifetime and to identify any risks (IVDR, Annex XIII, Part B, 4).
What to expect from BIOMEX
As a renowned regulatory expert and contract research organization (CRO), we provide professional support at every step of the performance evaluation process and also offer a variety of studies that are important for evaluation.
For example, with regard to analytical performance under IVDR, we offer testing of all relevant Annex I parameters such as analytical sensitivity, analytical specificity, trueness, precision, and accuracy.
Also, in the area of clinical performance, we provide all studies testing Annex I parameters, such as diagnostic sensitivity, diagnostic specificity, positive and negative predictive value, likelihood ratio, and expected values in normal and affected populations.
Analytical performance studies
Analytical performance is the basis for the clinical performance of a device and focuses on the analyte. Indicators of analytical performance are typically similar or even identical across IVD devices, and a number of Clinical & Laboratory Standards Institute (CLSI) documents provide guidance. BIOMEX offers studies for several scenarios, including:
Endogenous and exogenous interference, e.g., CLSI EP07-A2, as well as cross-reactivity, e.g., CLSI EP07-A2
Limit of Detection (LoD) for qualitative and quantitative tests, e.g., CLSI EP17-A2, Limit of Quantification (LoQ) for quantitative tests, e.g., CLSI EP17-A2, and Limit of Blank (LoB) required for quantitative tests and not listed in the IVDR, e.g., CLSI EP17-A2
- Measuring range for qualitative and quantitative tests
- Linearity for qualitative and quantitative tests, e.g., CLSI EP06A
- Cut-off for qualitative and quantitative tests, e.g., CLSI EP24-A2
- Trueness (bias), e.g., CLSI EP06-A or CLSI EP09-A3
- Precision (repeatability and reproducibility), e.g., CLSI EP05-A3
- Accuracy, resulting from trueness and precisions, e.g., CLSI EP21-A
- Validation of specimen collection
- Hook effect for immunological tests for qualitative tests – for quantitative tests it will be already included when measuring the range
- Sample stability evaluation (freeze/thaw cycles, matrices, specimen type), e.g., CLSI EP25-A
- Shipping and transport stability (only accelerated study)
- Shelf life (only accelerated study)
- Robustness studies (flex studies)
- Carry-over/cross-contamination for multiple use devices, e.g., CLSI H26-A2
Clinical performance studies
A clinical performance study focuses on the patient. Depending on your product, demonstrating clinical performance may require different types of studies. BIOMEX offers studies for several scenarios, including:
Clinical performance studies are to establish or confirm aspects of device safety and performance which cannot be determined by literature and/or previous experience gained by routine diagnostic testing.
Near-patient-testing or point-of-care testing means carrying out a test by healthcare professionals in hospitals or in physicians’ offices using a device in the presence of the patient without the need to send a sample to a laboratory.
Products that are to be used by laypersons for self-testing must comply with certain specifications, e.g., in terms of safety, applicability, and significance of their results.
Do you want more information about the studies we offer? Please check our FAQ page or contact us.
How we conduct your study – together with you
We know that every customer project is different. To convert challenges into success stories, here is how we work:
1
The meeting
In an online meeting – or together on site at our headquarters in Heidelberg – we will discuss the project together.
2
Expert check
Afterwards, BIOMEX’s experts will check the feasibility of your project, the capacity of staff and infrastructure, and the availability of all samples.
3
The offer
We will provide an individual offer for the study, including duration and costs.
4
Ready to go
As soon as the contract is signed, you are ready to go: We will create a study plan and coordinate it with you.
5
Collection and sample preparation
Depending on study requirements, collection and sample preparation will start. If necessary, we search our databases for suitable donors and plan a prospective sample collection.
6
Study start
Laboratory staff is trained and required reagents are prepared – and after an internal kick-off meeting, we can get started: The study begins.
7
Sampling & testing
The study is underway: Sampling is in progress. Your device is being tested, including reference testing, which involves comprehensive quality control.
8
Analysis
We manage and analyze the data so that you receive a preliminary study report for revision.
9
Study report
Upon completion of the study, you will receive the final study report.
Your Experts for Diagnostic Studies
You want to bring your assay to market as quickly as possible? We can help you with this – with our years of experience in diagnostic assay validation, broad regulatory expertise, large in-house biospecimens inventory, and our own donations centers.


